Soap
I’m guessing that the three most important health improvements in a modern (or emerging) society are water, sewers, and soap:
- Clean water: without it we die.
- Sewers: if we live in a city, sewers are necessary to get rid of human (and animal) waste and pathogens as well as helping eliminate food sources for disease-carrying rodents.
- Soap: the chemistry is a bit difficult but the actual process to make a basic soap product is straightforward.
Clean water and good sewers are fairly straightforward, although expensive in a community larger than a few people. But soap can easily be made, the most difficult part being a cooking vessel (think iron, ceramic or even hot rocks in a hollowed out log).
There are three steps in making soap:
- Collect wood ash and use to make lye.
- Collect fat and render it into tallow (cows or deer) or lard (pig).
- Combining the tallow/lard and lye over heat to make a soft soap.
Lye
Wood ash is the powdery reside left in the fireplace. The best ash ashes are from hard woods.
The ashes are soaked in chemical-free water (rainwater). The water flushes out the potassium (one of the elements found in the wood ash). The resulting liquid is caustic, specifically potassium hydroxide.
The soaking is considered done (strong enough) when the liquid is dense enough to float an egg. Note: dispose of the egg.
If not strong enough, the liquid will need to be boiled down to increase the density.
Rendering Fat
Add fat chunks from butchering to a heavy pot with water. The water is just to prevent burning and to help float the tallow/lard. Boil a low temperature (barely bubbling) for an hour or so. The tallow/lard will float on the water (any lighter than water impurities may need to be skimmed off).
Allow to cool. Then remove the white silky tallow/lard from the surface. You may need to scrape off any residue on the bottom surface of the tallow/lard.
Making Soap
Place the lye in a non-reactive pot (lye will dissolve an aluminum pot). Heat until it simmers.
Slowly add melted tallow/lard to the lye pot.
Heat at a simmer for several hours. Stir. Remove heat briefly if the pot starts to foam over.
At first, you’ll get an oily foam on top. After an hour or so, the liquid will thicken. Keep stirring.
Once homogeneous, remove from heat and allow to cool.
Now wash up! You now have a soft soap. (Hard soaps require Sodium Hydroxide, soft are made from Potassium Hydroxide which is in the wood ashes).
Some chemical notes (from Wikipedia)
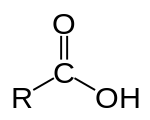
Soap is a salt of a fatty acid.
In chemistry, salts are ionic compounds that result from the neutralization reaction of an acid and a base. They are composed of related numbers of cations (positively charged ions) and anions (negative ions) so that the product is electrically neutral (without a net charge). These component ions can be inorganic, such as chloride (Cl−), or organic, such as acetate (C2H3O2−); and can be monatomic, such as fluoride (F−), or polyatomic, such as sulfate (SO42−).
There are several varieties of salts. Salts that hydrolyze to produce hydroxide ions when dissolved in water are basic salts, whilst those that hydrolyze to produce hydronium ions in water are acidic salts. Neutral salts are those that are neither acid nor basic salts. Zwitterions contain an anionic centre and a cationic centre in the same molecule, but are not considered to be salts. Examples include amino acids, many metabolites, peptides, and proteins.
A fatty acid is a carboxylic acid with a long aliphatic tail (chain), which is either saturated or unsaturated.
Potash is any of various mined and manufactured salts that contain potassium in water-soluble form. The name derives from “pot ash”, which refers to plant ashes soaked in water in a pot, the primary means of manufacturing the product before the industrial era. The word “potassium” is derived from potash.
Other References
Mother Earth News…making soap from ashes
Making It: Radical Home Ec for a Post-Consumer World
Any clarifications or corrections from you, the readers, are appreciated!